Introduction
While the broadness of the pyrolysis profile of most kerogens is described well by a parallel reaction model, the pyrolysis profile at a constant heating rate for certain well-preserved algal kerogens is narrower than can be described by a single first-order reaction or a single nth-order reaction. Pyrolysis data for GGU24, a well-preserved algal kerogen, is a prime example of this. Its narrow pyrolysis profiles are fit well by a nucleation-growth model.
In this example, we use Pyromat® data measured for GGU24 [Alan K. Burnham, Robert L. Braun, Thomas T. Coburn, Erik I. Sandvik, David J. Curry, Birthe J. Schmidt, and Rohinton A. Noble, An Appropriate Kinetic Model for Well-Preserved Algal Kerogens”, Energy & Fuels, Vol. 10, No. 1, pp. 49-59, 1996].
The files used in Case 4 for GGU24 can be downloaded as: ggu24.zip. All included files are text files and should be extracted to a user data folder that is not a subfolder of C:\Program Files.
GGU24 Pyrolysis data
| GGU2401.dat | Nominal heating rate of 1 C/min |
| GGU2406.dat | Nominal heating rate of 6 C/min |
| GGU2450.dat | Nominal heating rate of 50 C/min |
Single 1st-order reaction
| GGU24-A.con | Command file |
| GGU24-A.out | Output file |
| GGU24-A.dup.out | Duplicate of output file |
Single nth-order reaction
| GGU24-B.con | Command file |
| GGU24-B.out | Output file |
| GGU24-B.dup.out | Duplicate of output file |
Nucleation-Growth Model (3-parameter)
| GGU24-C.con | Command file |
| GGU24-C.out | Output file |
| GGU24-C.dup.out | Duplicate of output file |
Nucleation-Growth Model (4-parameter)
| GGU24-D.con | Command file |
| GGU24-D.out | Output file |
| GGU24-D.dup.out | Duplicate of output file |
Kinetics Analysis with 1st-Order Model
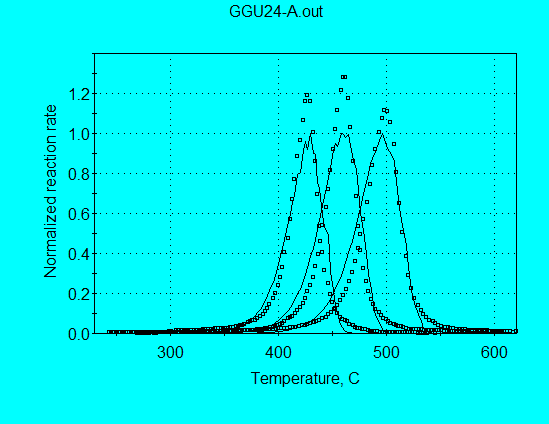
One of the simplest models for kinetics analysis is a 1st-order reaction model. Such a simple model does not fit most petroleum source rocks, although we illustrated in Case 3 that Kukersite is one source rock whose pyrolysis data are almost fit by this simple model. The pyrolysis profiles for some kerogens are even narrower than predicted by a single 1st-order reaction, as illustrated in the following graph for GGU24 (using the analysis command file GGU24-A.con).
The optimized kinetic parameters are:
A = 3.52 E+15 s-1
E = 59.58 kcal/mol
These abnormally high values of A and E result from the atttempt of the nonlinear regression to make the calculated profile narrower.
Kinetics Analysis with Nth-Order Model
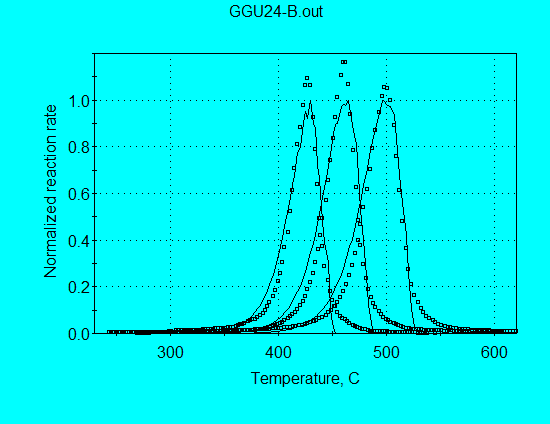
A simple improvement over the 1st-order reaction model is sometimes given by the nth-order reaction model. We illustrated in Case 3 that such a model fit Kukersite pyrolysis data quite well with a reaction order n = 1.238. Application of a single nth-order reaction for analysis of GGU24 pyrolysis data (using command file GGU24-B.con), however, is not as successful:
The optimized kinetic parameters are:
A = 6.39 E+14 s-1
E = 57.25 kcal/mol
n = 0.766
Reducing the reaction order to n = 0.766 narrows the calculated reaction profiles, giving good agreement of calculated and measured FWHH of the reaction profiles compared with those calculated for n = 1. However, the calculated reaction rate for n < 1 characteristically decreases rapidly in the latter stages of the reaction, thereby requiring higher than needed initial reaction rates for the optimized fit. Therefore, the overall fit of the nth-order reaction is poor.
Kinetics Analysis with Nucleation-Growth Model
Pyrolysis data for GGU24 are fit best by a nucleation-growth model. This model requires a frequency factor (A), a single activation energy (E), a nucleation exponent (m), and a reaction order (n). Analylsis of the GGU24 pyrolysis data with fixed n = 1 (using the analysis command file GGU24-C.con) gives the following optimized kinetic parameters:
A = 3.77 E+14 s-1
E = 55.91 kcal/mol
m = 0.283
The measured and calculated reaction profiles are in good agreement, except during the final stages of the reaction.
The agreement can be improved significantly by allowing all four parameters to be optimized (using the analysis command file GGU24-D.con). The optimized parameters are:
A = 5.23 E+14 s-1
E = 56.00 kcal/mol
m = 0.423
n = 1.276
The measured and calculated reaction profiles are now in good agreement over the entire extent of reaction.